Acquired Reactive Perforating Collagenosis: Association with Renal and Heart Failure
Matilda Bylaite and Thomas Ruzicka
Department of Dermatology, Heinrich-Heine-University Duesseldorf, Duesseldorf, Germany
History
A 68-year-old woman presented with a 2-week history of painful, rapidly enlarging oral lesions, noticed one week after the laser surgery of her right eye. Her medical history was significant for pyoderma gangrenosum, which was first diagnosed 5 years ago when the patient presented with ulcers on the dorsal aspects of both hands. Immunosuppressive therapy with oral corticosteroids and ciclosporin provided a rapid healing of ulcers. Despite maintaining immunosuppressive treatment, 2 years later, she had a short episode of ulcerating esophagitis, and a year later she re-presented with an ulcer involving her left medial malleolus. She responded well to the combined immunosuppressive therapy, including prednisolone 12.5 mg/day, ciclosporin 100 mg/day and mycophenolate mofetil 1500 mg/day. During the last 2 years, the doses of immunosuppressants were reduced to 10 mg/day prednisolone and 500 mg/day mycophenolate mofetil with no recurrence of skin lesions. The patient was continuously followed-up for possible underlying disorders. Moreover, a year ago, a sensomothoric polyneuropathy as a result of degenerative spinal disease was diagnosed. In addition, she had a history of dyslipidemia and hypertension and was regularly taking simvastatin and enalapril. Clinical Findings
On examination, numerous 0.5- to 3-cm erythematous plaques with distinctive central umbilication, containing a dark-brown, firmly adherent hyperkeratotic plug showing necrosis, and excoriations were distributed over her upper back, trunk, and on the extensor surface of her arms, knees and lateral aspects of her ankles. The lesions on the right ankle were ulcerated. There was no involvement of mucous membranes. Regional lymph nodes were not enlarged. A subcutaneous pacemaker generator was present in the right pectoral region.
| Fig. 1. Multiple lesions of acquired reactive perforating collagenosis (ARPC) on the back and extensor surfaces of the arms of 72-year-old woman. |
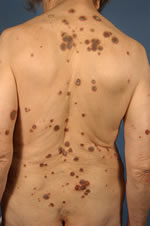 |

  |
| Fig. 2. Each ARPC plaque has a distinctive central umbilication, containing dark-brown, firmly adherent hyperkeratotic plug in part with necrosis and excoriations. |
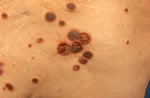 |

  |
Histopathology
The biopsy specimen showed a lesion with a central cup (bowl)-shaped crater extending from the epidermis to the reticular dermis, containing a mixture of parakeratotic material, basophilic degenerated fibers and inflammatory infiltrate, consisting mainly of lymphocytes, neutrophils and histiocytes (Fig. 3). Masson’s trichrome stain, which is not shown here, identified the perforating fibers to be collagen strands. Van Gieson staining for elastic fibers was negative.
| Fig. 3. Transepidermal elimination of degenerated collagen into a cup- (bowl)-shaped epidermal depression in ARPC (HE x40). |
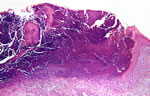 |

  |
Examination and Laboratory Findings
Initial investigations showed mild anemia (erythrocytes 3.61 M/dl, hemoglobin 11.7 g/dl, hematocrit 36.2%), leukocytosis (19.5 K/µl), markedly elevated CRP (243.8 mg/dl), uremia (195 mg/dl), elevated creatinine level (2.76 mg/dl), elevated uric acid level (93 mg/dl), elevated liver function tests (GOT 60 U/l, GPT 43 U/l, γ-GT 777 U/l), hypertriglyceridemia (284 mg/dl), mild hyperglycemia and normal electrolytes. Other biochemical parameters were within normal ranges.
Syphilis serology (TPPA) was negative. Anti-HBc-IgG was positive. The ANA titer was 1:640 with a speckled pattern of immunofluorescence; ANCA were negative.
Diagnostic tests for scabies were negative. Microbiologic examination of a swab from an ulcerated lesion from the right ankle showed the presence of Enterobacteriaceae. Chest X-ray and computed tomographic scan of the abdomen revealed no malignancy. Diagnosis
Based on history, clinical presentation and characteristic histological findings, a diagnosis of acquired reactive perforating collagenosis associated with renal and heart failure was established. Therapy and Course
Our patient was treated with oral prednisolone (80 mg/day tapered to 25 mg/day within 1 month), antihistamines, potent topical steroid cream (triamcinolone) and keratolytics. Steroid-induced moderate hyperglycemia was handled with short-acting insulin. The patient continued to receive her previous medications including oral allopurinol 300 mg/day for hyperuricemia. The treatment improved the patient’s condition by reducing pruritus; however, the skin lesions persisted and some even enlarged. UVB phototherapy, as an additional therapeutic option, was planned. After 4 weeks of therapy, our patient’s condition deteriorated with severe dyspnea, arrhythmia and decompensation of heart failure; therefore, she was referred to cardiologists and transferred to another clinic. Unfortunately, the contact with the patient was lost and further dermatological follow-up was impossible. Discussion
Pyoderma gangrenosum (PG) is a severe ulcerating noninfectious neutrophilic dermatosis, first described by Brunsting et al. in 1930 (1). Often PG ulcers are initiated by trauma (phenomenon of pathergy), and begin as tender papules, pustules, or hemorrhagic vesicles, which rapidly progress to large painful ulcers, having an undermined violaceous inflammatory border and a necrotic base (1-3). The most common sites affected are the lower extremities, buttocks, abdomen, and face (2-4). Although PG may occur at any age, the peak incidence is between 30 and 50 years (2). In more than 50 % of cases, there is an association with systemic diseases, such as Crohn’s disease, ulcerative colitis, rheumatoid arthritis, systemic lupus erythematosus, hepatitis or hematological malignancies (3, 4). The etiopathogenesis of PG remains obscure. The association with a number of immunologic disorders indicates that PG is related to various underlying defective immune mechanisms, including abnormalities of neutrophil function, T helper-suppressor cell imbalance and impaired phagocytosis (5). Oral PG has been rarely described (6-9). In most reported cases, there was an association with cutaneous lesions; however, PG might exclusively affect the oral mucosa (9). Predilection sites of mucosal involvement are tongue, palate and tonsilar fauces (6-9). The lesions are usually painful, irregularly shaped with rolled out margins and a gray-colored base. In most of the previous reported cases, oral PG was also associated with inflammatory bowel disease and hematological disorders. We did not detect any of these underlying disorders in our patient. The histopathology of oral PG is not specific. It shows chronic ulceration and neutrophilic-rich infiltration of the lamina propria and dense inflammatory cells infiltration of submucosa, with or without signs of vasculitis (3, 4). Microbiological examination of swabs from the ulcers is sterile in up to 50% of cases (3). The differential diagnosis of oral PG includes squamous cell carcinoma, pyostomatitis vegetans, infectious conditions and other neutrophilic dermatoses, such as Sweet’s syndrome and Behçet’s disease (9). The optimal treatment of PG consists of elimination of disease activity, reduction of pain, local wound care and treatment of underlying associated diseases (4). To date, high doses of systemic corticosteroids (1-2 mg/kg/day) are the mainstay of therapy. Ciclosporin (3-5 mg/kg/day) has been shown to be effective both alone and in combination with systemic corticosteroids (3, 4). Sulfa drugs, such as dapsone, sulfapyridine and sulfasalazine might be beneficial in PG. Other treatment modalities include azathioprine, methotrexate, cyclophosphamide, minocycline, clofazimine and colchicine (3, 4, 8, 10). Recently, topical and systemic tacrolimus (FK506) has been successfully used in management of PG (11, 12). Treatment with intravenous immunoglobulin, thalidomide, plasmapheresis and mycophenolate mofetil (13) has also been used especially in resistant PG cases. For extreme cases of PG, surgical intervention might be required (14). In the past few years infliximab, a chimeric monoclonal anti-TNF-α antibody, alone or in combination with other immunosuppressive agents has shown a great promise in treating a wide range of inflammatory conditions including refractory PG (15, 16). Infliximab inhibits the activity of TNF-α, which is important in the pathophysiological process of immune-mediated inflammatory disorders. Despite all efforts and recent advances, the treatment of PG remains a challenge. The prognosis is also unpredictable since the lesions often reoccur with cessation of immunosuppressive agents. Furthermore, careful monitoring of patients with PG for underlying systemic disease is required because of high risk of malignant transformation. Herein we describe a 68-year-old woman who presented with severe oral pyoderma gangrenosum and showed excellent response to infliximab as adjunct therapy to prednisolone and mycophenolate mofetil. Rapid recognition of various PG presentations and initiation of aggressive immunosuppressive therapy is important for its potential destructive and disabling behavior. References
1. Brunsting, L.A., Goeckerman, W., O’Leary, P.A. Pyoderma (ecthyma) gangrenosum: clinical and experimental observations in five cases occuring in adults. Arch Dermatol 1930, 22: 655-80. 2. Powell, F.C., Schroeter, A.L., Su, W.P.D. et al. Pyoderma gangrenosum: a review of 86 patients. Q J Med 1985, 55: 173-86. 3. Von den Driesch, P. Pyoderma gangrenosum: a report of 44 cases with long follow-up. Br J Dermatol 1997, 137: 1000-5. 4. Bennett, M.L., Jackson, J.M., Jorizzo, J.L., Fleischer, A.B. Jr, White, W.L., Callen, J.P. Pyoderma gangrenosum, a comparison of typical and atypical forms with emphasis on time to remission: case review of 86 patients from 2 institutions. Medicine 2000, 79: 37-46. 5. Wolff, K., Stingl, G. Pyoderma gangrenosum. In: Dermatology in General Medicine, 5th ed. I.M. Freedberg, A.Z. Eisen, K. Wolff et al. (Eds.). McGraw Hill: New York 1999, 1140-8. 6. Margoles, J.S., Wegner, J. Stomal ulceration associated with pyoderma gangrenosum and ulcerative colitis. Report of two cases. Gastroenterology 1961, 41: 594-8. 7. Yco, M.S., Warnock, G.R., Cruickshank, J.C., Burnett, J.R. Pyoderma gangrenosum involving the head and neck. Laryngology 1988, 98: 765-8. 8. Setterfield, J.F., Shirlaw, P.J., Challacombe, S.J., Black, M.M. Pyoderma gangrenosum associated with severe oropharyngeal involvement and IgA paraproteinemia. Br J Dermatol 2001, 144: 393-6. 9. Martin, A.H., Palomo, D.A., Hermida, G. et al. Oral pyoderma gangrenosum. Br J Dermatol 2003, 149: 663-4. 10. Chow, R.K.P., Ho, V.C. Treatment of pyoderma gangrenosum. J Am Acad Dermatol 1996, 34: 1047-60. 11. Baumgart, D.C., Wiedenmann, B., Dignass, U. Successful therapy of refractory pyoderma gangrenosum and periorbital phlegmona with tacrolimus (FK506) in ulcerative colitis. Iinflamm Bowel Dis 2004, 10: 421-4. 12. Schuppe, H.C., Homey, B., Assmann, T., Martens, R., Ruzicka, T. Topical tacrolimus for pyoderma gangrenosum. Lancet 1998, 351: 832. 13. Nousari, H.C., Lynch, W., Anhalt, G.J. et al. The effectiveness of mycophenolate mofetil in refractory pyoderma gangrenosum. Arch Dermatol 1998, 134: 1509-11. 14. Alarm, M., Grossman, M.E., Schneiderman, P.I. et al. Surgical management of pyoderma gangrenosum: case report and review. Dermatol Surg 2000, 26: 1063-6. 15. Tan, M.H., Gordon, M., Lebwohl, O., George, J., Lebwohl, M.G. Improvement of pyoderma gangrenosum and psoriasis associated with Crohn’s disease with anti-tumor necrosis factor alpha monoclonal antibody. Arch Dermatol 2001, 137: 930-3. 16. Regueiro, M., Valentine, J., Plevy, S., Fleisher, M.R., Lichtenstein, G.R. Infliximab for treatment of pyoderma gangrenosum associated with inflammatory bowel disease. Am J Gastroenterol 2003, 98: 1821-6.
|