Oral Pyoderma Gangrenosum: Response to Infliximab
Matilda Bylaite and Thomas Ruzicka
Department of Dermatology, Heinrich-Heine-University Duesseldorf, Duesseldorf, Germany
History
A 68-year-old woman presented with a 2-week history of painful, rapidly enlarging oral lesions, noticed one week after the laser surgery of her right eye. Her medical history was significant for pyoderma gangrenosum, which was first diagnosed 5 years ago when the patient presented with ulcers on the dorsal aspects of both hands. Immunosuppressive therapy with oral corticosteroids and ciclosporin provided a rapid healing of ulcers. Despite maintaining immunosuppressive treatment, 2 years later, she had a short episode of ulcerating esophagitis, and a year later she re-presented with an ulcer involving her left medial malleolus. She responded well to the combined immunosuppressive therapy, including prednisolone 12.5 mg/day, ciclosporin 100 mg/day and mycophenolate mofetil 1500 mg/day. During the last 2 years, the doses of immunosuppressants were reduced to 10 mg/day prednisolone and 500 mg/day mycophenolate mofetil with no recurrence of skin lesions. The patient was continuously followed-up for possible underlying disorders. Moreover, a year ago, a sensomothoric polyneuropathy as a result of degenerative spinal disease was diagnosed. In addition, she had a history of dyslipidemia and hypertension and was regularly taking simvastatin and enalapril. Clinical Findings
The oropharyngeal examination revealed two 1.8 x 3 cm and 0.5 x 0.8 cm, irregularly shaped but well-circumscribed ulcers on her right buccal mucosa (Fig. 1), and 1.5 x 2.5 cm ulcer involving the hard and soft palate (Fig. 2). Each oral ulcer had a thick, gray-colored necrotic base surrounded with erythematous border. There was no involvement of other mucous membranes. She had no cutaneous lesions, except for few irregular scars on the dorsal aspects of her hands and left medial malleolus, indicating healed previous ulcers. The regional lymph nodes were enlarged. Systemic examination was within normal limits.
| Fig. 1. Severe necrotic ulcerations of pyoderma gangrenosum in the right buccal mucosa. |
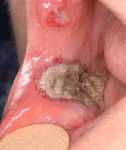 |

  |
| Fig. 2. Extensive necrotic ulceration of pyoderma gangrenosum involving the hard and soft palate. |
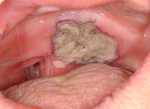 |

  |
Histopathology
Histopathological examination of several oral biopsy specimens showed an ulcerated hyperplastic and spongiotic mucosa with a dense neutrophilic infiltrate in the lamina propria and a mixed inflammatory cell infiltrate in the submucosa without signs of vasculitis (Fig. 3). Periodic acid-Schiff and Gram stains were negative.
| Fig. 3. Oral pyoderma gangrenosum: neutrophilic exocytosis and a dense inflammatory neutrophil-rich infiltrate within the lamina propria and with no signs of vasculitis (HE x100). |
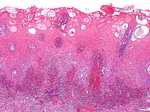 |

  |
Examination and Laboratory Findings
Laboratory tests disclosed the following abnormal parameters: leukocytes 12.9 K/ml (N: 4-11), hemoglobin 11.6 g/dl (12-16), total cholesterol 277 mg/dl (N: <200), a2-globulin 17.8% (7.6-13.4) and g-globulin 8.5% (9.7-17.9). She had positive serological results for hepatitis B. Remaining laboratory parameters were within normal limits or negative. Microbiological culture of swabs from the oral ulcers failed to show any growth of pathogenic organisms. Abdomen ultrasonography, esophagogastroduodenoscopy, chest X-ray and skeletal scintigraphy were unremarkable. Colonoscopy revealed few tubular adenomas having a low-grade dysplasia in the transversal colon and cecum, which were removed during the procedure. No other associated systemic disease or malignancies were identified. Diagnosis
Based on history, clinical and histopatological findings, a diagnosis of oral pyoderma gangrenosusm was made. Therapy and Course
The patient was initially treated with systemic immunosuppressants, including prednisolone 80 mg/day and mycophenolate mofetil 2 g/day. Topical antiseptics, hexetidine and tacrolimus (Prograf 1%) mouth rinse were administered. In addition, she was taking paracetamol, omeprazole and nifedipine. However, there was no response after 1 month of combined immunosuppressive therapy. The patient could not eat and lost weight due to continuously enlarging and increasingly more painful oral ulcers. Additional therapy with infliximab 5 mg/kg, according to a recommended standard protocol, was initiated. Her condition improved dramatically within the following 2 weeks, showing an evident healing of oral lesions. An excellent response to the combined treatment without side effects was observed after 3 infusions (Figs. 4 and 5). Presently, the patient is maintained on oral prednisolone 20 mg/day and mycophenolate mofetil 2 g/day and scheduled for 8-week-interval infliximab therapy.
| Fig. 4. Subsiding lesions of oral pyoderma gangrenosum after 3 infusions of infliximab in conjunction with prednisone and mycophenolate mofetil therapy. |
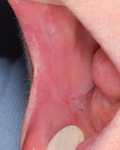 |

  |
| Fig. 5. Remarkable regression of oral pyoderma gangrenosum ulcer after initiation of infliximab. |
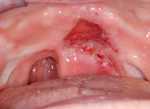 |

  |
Discussion
Pyoderma gangrenosum (PG) is a severe ulcerating noninfectious neutrophilic dermatosis, first described by Brunsting et al. in 1930 (1). Often PG ulcers are initiated by trauma (phenomenon of pathergy), and begin as tender papules, pustules, or hemorrhagic vesicles, which rapidly progress to large painful ulcers, having an undermined violaceous inflammatory border and a necrotic base (1-3). The most common sites affected are the lower extremities, buttocks, abdomen, and face (2-4). Although PG may occur at any age, the peak incidence is between 30 and 50 years (2). In more than 50 % of cases, there is an association with systemic diseases, such as Crohn’s disease, ulcerative colitis, rheumatoid arthritis, systemic lupus erythematosus, hepatitis or hematological malignancies (3, 4). The etiopathogenesis of PG remains obscure. The association with a number of immunologic disorders indicates that PG is related to various underlying defective immune mechanisms, including abnormalities of neutrophil function, T helper-suppressor cell imbalance and impaired phagocytosis (5). Oral PG has been rarely described (6-9). In most reported cases, there was an association with cutaneous lesions; however, PG might exclusively affect the oral mucosa (9). Predilection sites of mucosal involvement are tongue, palate and tonsilar fauces (6-9). The lesions are usually painful, irregularly shaped with rolled out margins and a gray-colored base. In most of the previous reported cases, oral PG was also associated with inflammatory bowel disease and hematological disorders. We did not detect any of these underlying disorders in our patient. The histopathology of oral PG is not specific. It shows chronic ulceration and neutrophilic-rich infiltration of the lamina propria and dense inflammatory cells infiltration of submucosa, with or without signs of vasculitis (3, 4). Microbiological examination of swabs from the ulcers is sterile in up to 50% of cases (3). The differential diagnosis of oral PG includes squamous cell carcinoma, pyostomatitis vegetans, infectious conditions and other neutrophilic dermatoses, such as Sweet’s syndrome and Behçet’s disease (9). The optimal treatment of PG consists of elimination of disease activity, reduction of pain, local wound care and treatment of underlying associated diseases (4). To date, high doses of systemic corticosteroids (1-2 mg/kg/day) are the mainstay of therapy. Ciclosporin (3-5 mg/kg/day) has been shown to be effective both alone and in combination with systemic corticosteroids (3, 4). Sulfa drugs, such as dapsone, sulfapyridine and sulfasalazine might be beneficial in PG. Other treatment modalities include azathioprine, methotrexate, cyclophosphamide, minocycline, clofazimine and colchicine (3, 4, 8, 10). Recently, topical and systemic tacrolimus (FK506) has been successfully used in management of PG (11, 12). Treatment with intravenous immunoglobulin, thalidomide, plasmapheresis and mycophenolate mofetil (13) has also been used especially in resistant PG cases. For extreme cases of PG, surgical intervention might be required (14). In the past few years infliximab, a chimeric monoclonal anti-TNF-α, which is important in the pathophysiological process of immune-mediated inflammatory disorders. Despite all efforts and recent advances, the treatment of PG remains a challenge. The prognosis is also unpredictable since the lesions often reoccur with cessation of immunosuppressive agents. Furthermore, careful monitoring of patients with PG for underlying systemic disease is required because of high risk of malignant transformation. Herein we describe a 68-year-old woman who presented with severe oral pyoderma gangrenosum and showed excellent response to infliximab as adjunct therapy to prednisolone and mycophenolate mofetil. Rapid recognition of various PG presentations and initiation of aggressive immunosuppressive therapy is important for its potential destructive and disabling behavior. References
1. Brunsting, L.A., Goeckerman, W., O’Leary, P.A. Pyoderma (ecthyma) gangrenosum: clinical and experimental observations in five cases occuring in adults. Arch Dermatol 1930, 22: 655-80. 2. Powell, F.C., Schroeter, A.L., Su, W.P.D. et al. Pyoderma gangrenosum: a review of 86 patients. Q J Med 1985, 55: 173-86. 3. Von den Driesch, P. Pyoderma gangrenosum: a report of 44 cases with long follow-up. Br J Dermatol 1997, 137: 1000-5. 4. Bennett, M.L., Jackson, J.M., Jorizzo, J.L., Fleischer, A.B. Jr, White, W.L., Callen, J.P. Pyoderma gangrenosum, a comparison of typical and atypical forms with emphasis on time to remission: case review of 86 patients from 2 institutions. Medicine 2000, 79: 37-46. 5. Wolff, K., Stingl, G. Pyoderma gangrenosum. In: Dermatology in General Medicine, 5th ed. I.M. Freedberg, A.Z. Eisen, K. Wolff et al. (Eds.). McGraw Hill: New York 1999, 1140-8. 6. Margoles, J.S., Wegner, J. Stomal ulceration associated with pyoderma gangrenosum and ulcerative colitis. Report of two cases. Gastroenterology 1961, 41: 594-8. 7. Yco, M.S., Warnock, G.R., Cruickshank, J.C., Burnett, J.R. Pyoderma gangrenosum involving the head and neck. Laryngology 1988, 98: 765-8. 8. Setterfield, J.F., Shirlaw, P.J., Challacombe, S.J., Black, M.M. Pyoderma gangrenosum associated with severe oropharyngeal involvement and IgA paraproteinemia. Br J Dermatol 2001, 144: 393-6. 9. Martin, A.H., Palomo, D.A., Hermida, G. et al. Oral pyoderma gangrenosum. Br J Dermatol 2003, 149: 663-4. 10. Chow, R.K.P., Ho, V.C. Treatment of pyoderma gangrenosum. J Am Acad Dermatol 1996, 34: 1047-60. 11. Baumgart, D.C., Wiedenmann, B., Dignass, U. Successful therapy of refractory pyoderma gangrenosum and periorbital phlegmona with tacrolimus (FK506) in ulcerative colitis. Iinflamm Bowel Dis 2004, 10: 421-4. 12. Schuppe, H.C., Homey, B., Assmann, T., Martens, R., Ruzicka, T. Topical tacrolimus for pyoderma gangrenosum. Lancet 1998, 351: 832. 13. Nousari, H.C., Lynch, W., Anhalt, G.J. et al. The effectiveness of mycophenolate mofetil in refractory pyoderma gangrenosum. Arch Dermatol 1998, 134: 1509-11. 14. Alarm, M., Grossman, M.E., Schneiderman, P.I. et al. Surgical management of pyoderma gangrenosum: case report and review. Dermatol Surg 2000, 26: 1063-6. 15. Tan, M.H., Gordon, M., Lebwohl, O., George, J., Lebwohl, M.G. Improvement of pyoderma gangrenosum and psoriasis associated with Crohn’s disease with anti-tumor necrosis factor alpha monoclonal antibody. Arch Dermatol 2001, 137: 930-3. 16. Regueiro, M., Valentine, J., Plevy, S., Fleisher, M.R., Lichtenstein, G.R. Infliximab for treatment of pyoderma gangrenosum associated with inflammatory bowel disease. Am J Gastroenterol 2003, 98: 1821-6. |