Eccrine Porocarcinoma, Bowen's Disease and Basal Cell Carcinomas in an Immunosuppressed Patient
Matilda Bylaite and Thomas Ruzicka
Department of Dermatology, Heinrich-Heine-University Duesseldorf, Duesseldorf, Germany
History
A 63-year-old woman was referred to our hospital for excision of asymptomatic, multiple tumors on her face and back, which had been slowly growing for 4 years. Within 3 months, two of the tumors on her face increased in size, eroded and started to bleed. Several biopsies were taken by her dermatologist, and histological features were consistent with Bowen’s disease. Her previous medical history included hysterectomy (30 years earlier) and breast cancer (20 years earlier), treated by right mastectomy and one course of chemotherapy. Moreover, multiple myeloma with overproduction of IgG Lambda was diagnosed 10 years later. In the following years, she has been in partial remission, regularly receiving numerous courses of different chemotherapy. There was no history of ultraviolet overexposure. The patient was admitted for further examination and tumor excision. Clinical Findings
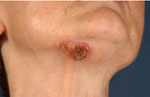
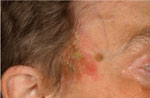
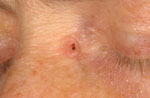
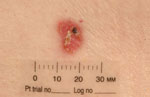
Physical examination revealed four different skin lesions. A 2x4 cm in diameter, asymmetrical, erythematous, indurated plaque with a slightly elevated border and a reddish, ulcerated, crusted 2.5 cm nodule was located on her right submental area (Fig. 1). A 5-cm flat, ill-demarcated, scaly, eroded erythematous plaque was observed in the right temporal area (Fig. 2). A well-defined, smooth, pearly tumor with telangiectasia and a slightly elevated border was noticed in a "danger zone" in the medial angle of the left eye (Fig. 3). A 1.5-cm erythematous, slightly raised plaque with a central crust was located in her right lumbar region (Fig. 4). Regional lymph nodes were not enlarged.
| Fig. 1: Eccrine porocarcinoma. Primary malignant tumor of eccrine sweat gland, arising from the intraepidermal ductal part. |
 |

  |
| Fig. 2: Bowen’s disease localized on the right temporal area. |
 |

  |
| Fig. 3: Nodular basal cell carcinoma localized in a “danger zone.” |
 |

  |
| Fig. 4: Superficial basal cell carcinoma localized on the patient’s back. |
 |

  |
Histopathology
Histological examination of the ulcerated tumor from her submental area showed irregularly shaped tumor nests from the epidermis to the dermis. They consisted of small, monomorphic, basaloid cells with eosinophilic cytoplasm and large pleomorphic nuclei. Several areas of necrosis, atypical mitoses and intracellular-duct-like lumina were seen. The histopathological features were consistent with eccrine porocarcinoma (Fig. 5). A skin biopsy from the right temporal region showed typical Bowen’s disease (Fig. 6). The tumor around the eye proved to be a solid basal cell carcinoma (Fig. 7). The plaque on her back was also diagnosed as solid basal cell carcinoma (Fig. 8).
| Fig. 5: Eccrine porocarcinoma. The irregularly shaped tumor nests, extending from the epidermis to the subcutaneous tissue, are composed of cells, showing anaplastic nuclei and dyskeratosis (HE stain. x40). Insert: Basaloid cells containing pleomorphic nuclei and atypical mitoses (HE stain. x400). |
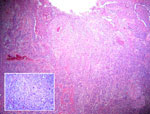 |

  |
| Fig. 6: Bowen’s disease (HE stain. x100). |
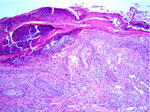 |

  |
| Fig. 7: Solid basal cell carcinoma (HE stain. x100). |
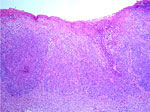 |

  |
| Fig. 8: Solid basal cell carcinoma (HE stain. x100). |
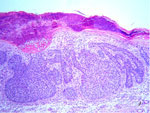 |

  |
Examination and Laboratory Findings
Results of laboratory investigations revealed moderate anemia (Hb 8.6 g/dl), slightly elevated CRP and increased concentration of total protein (13.4 g/dl) with overproduction of gamma globulin (8.6 g/dl). Staging examinations revealed no metastasis except for multiple punched-out osteolytic lesions of the bones. Diagnosis
Based on the clinical and histopathological findings, the diagnosis of an eccrine porocarcinoma, Bowen’s disease and two basal cell carcinomas in our immunosuppressed patient with multiple myeloma and a history of breast cancer was established. Therapy and Course
Eccrine porocarcinoma was completely excised using Moh’s micrographic surgery and a skin flap. Two basal cell carcinomas on the back and face were also removed by Moh’s surgery. The Bowen’s disease was treated with cryosurgery. Neither chemotherapy nor radiotherapy for eccrine porocarcinoma was instituted. The standard chemotherapy for multiple myeloma was continued. Discussion
Eccrine porocarcinoma (EP) is a very rare malignant tumor of eccrine sweat gland, arising from the intraepidermal ductal part, "acrosyringium." Pinkus and Mehregan first reported it in 1963 as an uncommon malignant adnexal tumor of the skin, characterized by epidermotropism with a pagetoid diffusion within epidermis (1). To date, more than 140 cases of EP have been reported in the literature under a variety of names such as dysplastic poroma, malignant syringoacanthoma, malignant eccrine poroma, malignant hydroacanthoma simplex, poroepithelioma or porocarcinoma. Eccrine porocarcinoma usually presents in elderly people and in both sexes almost equally. The mean age of onset is around 65 years and the mean length of time from initial tumor presentation to treatment is 8.5 years (2). The tumor arises from a preexisting benign eccrine poroma, actinic lesions and chronic lymphatic leukemia or develops de novo (2). The clinical picture of eccrine porocarcinoma is nonspecific. Patients usually present with solitary, well-circumscribed, slowly growing, asymptomatic, erythematous papule, nodule or plaque of about 1-10 cm, which can grow in a polypoid, verrucous pattern or ulcerate (3, 4). In more than 50% of reported cases, the tumor is found on the lower extremities. Other common sites include face, scalp and ear (about 20%), upper extremities (about 11%), abdomen and trunk (about 9%) and genital sites (5). The diagnosis of eccrine porocarcinoma is based on characteristic histopathological findings. Monomorphic, glycogen-rich, PAS-positive basaloid cells with large, irregularly shaped nuclei, hyperchromatic mitoses and eosinophilic basement membrane-like material may be observed (3). The malignant cells may be limited to the epidermis, and later the tumor may extend to the dermis, subcutaneous fat and lymphatic system (3, 5, 8). Two histopathological variants of EP are described: a trabecular variant and a more aggressive epidermotropic variant. Immunohistochemistry with positive staining for p53, carcinoembryonic (CEA) or epithelial membrane (EMA) antigens may confirm the diagnosis. Clinically, eccrine porocarcinomas are often mistaken for squamous cell carcinoma, basal cell carcinoma, granuloma teleangiectaticum, fibroma or a cutaneous metastasis of breast or visceral carcinomas (3, 5). Pathologically, identification of eccrine porocarcinoma may also be difficult and it may be confused with inflamed poroma, hydradenocarcinoma, Bowen’s or Paget’s disease, dermal duct tumor, metastatic breast carcinoma, basal cell carcinoma or squamous cell carcinoma (6). Primary treatment of eccrine porocarcinoma should consist of a wide surgical excision of the tumor and regional lymphadenectomy, if indicated, with an 80% cure rate (5). The value of adjunctive therapy has not been proved. Several chemotherapy protocols and radiation treatments have been used with variable response rates (2, 5, 8) and some benefit has been observed in a few cases with alpha interferon and retinoids (9). The local recurrence and regional metastatic rates are both approximately 20%. Distant metastases occur in around 12% of cases. The most common metastatic sites include lymph nodes, lung, retroperitoneum, peritoneum, femur, breast, liver, bladder, ovary and adrenal glands (5-7). The mortality rate is more than 65% when regional nodes are involved (5). In our patient, an eccrine porocarcinoma was associated with Bowen’s disease, two basal cell carcinomas, multiple myeloma and a history of breast cancer. Multiple skin carcinomas are not uncommon and can be observed in patients with genetic defects, such as Gorlin syndrome or xeroderma pigmentosum, in patients previously exposed to arsenic, hydrocarbons, photochemotherapy or x-ray therapy, in immunosuppressed patients, as in our case, or for unknown reasons. Although there was no evidence of metastasis of eccrine porocarcinoma in our patient, a long-term follow-up remains essential after a wide excision of the tumor. References
1. Pinkus, H., Mehregan, A. Epidermotropic eccrine carcinoma: A case combining features of eccrine poroma and Paget’s dermatosis. Arch Dermatol 1963, 88: 597-607.
2. Ritter, A.M., Graham, R.S., Amaker, B., Broaddus, W.C., Young, H.F. Intracranial extension of an eccrine porocarcinoma. Case report and review of the literature. J Neurosci 1999, 90: 138-40.
3. Shaw, M., McKee, P., Lowe, D., Black, M.M. Malignant eccrine poroma: A study of 27 cases. Br J Dermatol 1982, 107: 675-80.
4. Mehregan, A.H., Hashimoto, K., Rahbari, H. Eccrine adenocarcinoma: A clinicopathological study of 35 cases. Arch Dermatol 1983, 119: 104-14.
5. Snow, S.N., Reizner, G.T. Eccrine porocarcinoma of the face. J Am Acad Dermatol 1992, 27: 306-11.
6. Lozano Orella, J.A., Valcayo Penalba, A., San Juan, C.C., Vives Nadal, R., Castro Morrondo, J., Tunon Alvarez, T. Eccrine porocarcinoma. Report of nine cases. Dermatol Surg 1997, 23: 925-8.
7. Ryan, J.F., Darley, C.R., Pollock, D.J. Malignant eccrine poroma: Report of three cases. J Clin Pathol 1986, 39: 1099-104.
8. Hyman, A.B., Brownstein, M.H. Eccrine poroma. An analysis of forty-five new cases. Dermatologica 1969, 138: 29-38.
9. Barzi, A.S., Ruggeri, S., Recchia, F., Bertoldi, I. Malignant metastatic eccrine poroma. Proposal for new therapeutic protocol. Dermatol Surg 1997, 23: 267-72. |